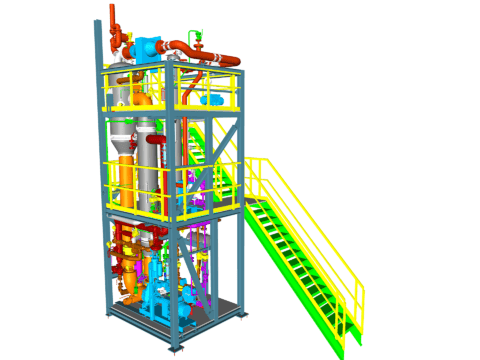
Crystallization of NaCl (Vacuum Salt)

The crystallization of NaCl into table salt (vacuum salt) or even higher-grade salt such as salt for the pharmaceutical industry can be achieved using different crystallization methods.
Typically, this process begins with a brine purification of the incoming raw solution, where impurities such as magnesium, calcium, or sulfate ions are precipitated. This can be done using the conventional brine purification method or the established Schweizer-Halle process.
The purified solution is then fed into an evaporation-crystallization plant.
In recent years, especially due to the costs of electrical energy and steam combined with low CO₂ emissions, the mechanical vapor recompression process has become the primarily used method.
Alternatively, crystallization can also take place in a multi-effect plant heated with fresh steam. In this plant, NaCl is crystallized in several stages, and the impurities still present after brine purification are concentrated from stage to stage. This method allows to produce different salt qualities by extraction of the salt from different stages, such as NaCl in pharmaceutical quality and classic vacuum salt as table salt, in one facility.
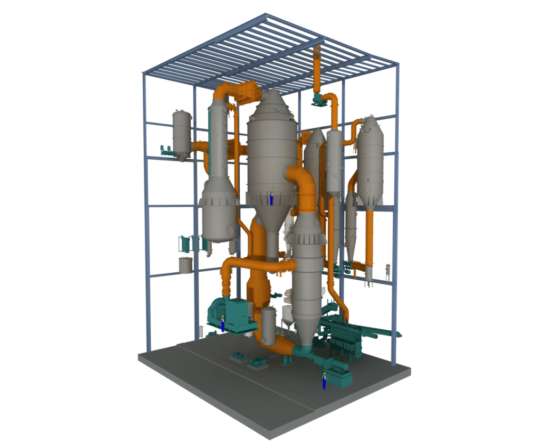
The mother liquor resulting from the centrifugation of NaCl salt is partly returned to brine purification in both methods mentioned above; and partly discharged from the system.
Depending on the composition of the solution after brine purification, it is also possible, instead of discharge, to undertake further steps to produce high-value fertilizers such as potassium sulfate (SOP).
To achieve the necessary quality parameters of the salt, a separate discharge of clear solution and thickened salt suspension has proven advantageous in the individual crystallizers.
This allows the crystal concentration within each circuit to be adjusted. Simultaneously, during the separation from the so-called salt leg, counterflow with fresh solution can also purify the moisture on the salt, thus increasing the salt purity after centrifuging and drying.


Another variant of vacuum salt production is vacuum cooling crystallization. This is particularly used when the desired salt quality requires lower specifications or the initial salt allows this due to its impurities.
In this process, the raw salt is dissolved in a saturated solution and circulated. Insoluble components are retained from the beginning and discharged from the plant.
The temperature dependency of NaCl’s solubility is utilized in vacuum cooling crystallization by gradually lowering the temperature and simultaneously removing water through evaporation cooling, crossing the saturation line and crystallizing NaCl salt.



EBNER – your competent partner in plant engineering
Thanks to many years of experience in various industrial sectors and with a wide range of solutions, EBNER is your contact for optimal process design, planning, delivery, and erection according to your specific needs.
Frequently Asked Questions
What methods can be used in the crystallization of NaCl?
For producing table salt and high-purity pharmaceutical salt, the mechanical vapor recompression and multi-effect plant methods have been established. However, depending on the requirements, the methods of thermal vapor recompression and vacuum cooling crystallization can also be used.
The choice of method also depends on the energy costs and product requirements.
Why is the brine partially purified before entering the crystallization plant?
This is necessary to free the solution from interfering ions. Certain contents tend to:
- cause scaling on the heating surfaces and increased cleaning requirements
- increase the energy consumption of the plant
- decrease the product quality
Why is part of the solution discharged from the plant despite the purification?
Typically, the solution also contains substances like Boron that cannot be precipitated in chemical purification or whose precipitation would be too costly. These substances accumulate in the circuit and reduce the product quality of the vacuum salt.
The only way to remove these is either by discharge or, if possible, further processing into valuable materials.
Other applications