Evaporation of Aluminate Solution

In the Bayer process for extracting bauxite with aqueous sodium hydroxide to produce aluminum oxide, a so-called thin liquor is generated during the process. This liquor, mainly consisting of NaOH and Al(OH)₃, is referred to as aluminate liquor or sodium aluminate liquor.
To reuse the liquor for the bauxite extraction process, it needs to be concentrated.
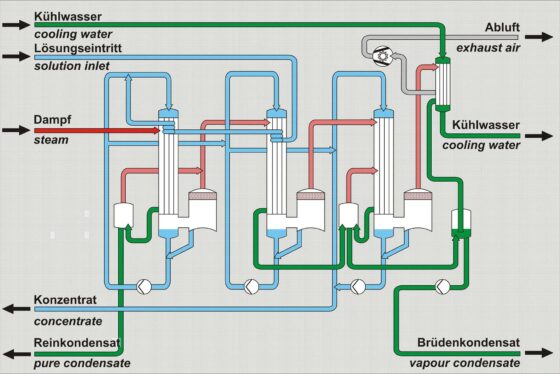
For evaporation, the principle of multi-effect evaporation in co-current flow and the principle of flash evaporation have predominantly been established.
Developments tend towards single-stage plants with compression, depending on the components of the solution and the desired evaporation degree. Evaporation plants with mechanical vapor recompression are possible, and plants with thermal vapor recompression have been realized.


Depending on the solution’s ingredients, the plants can either be designed as falling film plants or, in cases of increased fouling behavior of the heat exchanger surfaces, as forced circulation plants.

EBNER – your competent partner in plant engineering
With many years of experience in various industries and with different solutions, EBNER is your contact for optimally executing process design, planning, delivery, and erection according to your specific needs.
Frequently Asked Questions
What should be considered in evaporation plants for aluminate liquor?
Evaporation plants for sodium aluminate liquors, or aluminate liquors in general, must be designed to avoid reaching the salt crystallization zone of the ingredients (e.g., NaAlO₂ and its hydrates or Na₂CO₃ and its hydrates).
Why aren’t all plants executed with falling film evaporation?
It varies from case to case, depending on the fouling behavior of the heat exchanger surfaces.
If fouling is expected, the zone of evaporation and thus the concentration of the solution should not occur on the heat-transferring tubes themselves, as is the case with falling film evaporators.
Plants with a separate heat transfer zone in a heat exchanger and the the evaporation zone in the evaporator are preferred.
Which substances can cause fouling?
Typically, (sodium) silicates, (sodium) carbonates, and calcium salts are responsible for fouling.
Other applications