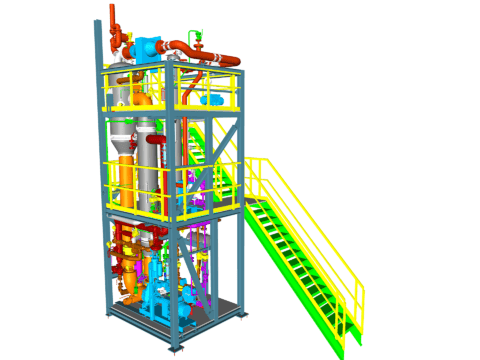
Crystallization of LiCl
One application in the entire lithium salt production is the crystallization of lithium chloride (LiCl).
Here, the crystallization of lithium chloride represents a direct step from evaporation to crystallization, so no intermediate steps, as are common with other lithium salts, need to be conducted.
The choice of the evaporation plant for LiCl solution and for the crystallization of LiCl salts is limited due to the high boiling point elevation of saturated LiCl solutions.
As a result, plants with mechanical vapor recompression are unsuitable, as many vapor compressors (fans) are necessary for the compression of the vapor, which significantly increases the investment costs of such a plant. Furthermore, high costs of electrical energy per ton of produced lithium chloride would also be arise in operation.
Due to the low energy efficiency of plants with thermal vapor recompression at such high boiling point elevations, combined with the contamination of fresh steam with vapor, thermal vapor compression is also practically not used.
As a resulting option, crystallization plants for LiCl are therefore mainly executed as multi-effect plants.

EBNER – your competent partner in plant engineering
With many years of experience in various industrial sectors and with a variety of solutions, EBNER is your contact for optimally executing the process design, planning, delivery, and erection according to your needs.
Frequently Asked Questions
What special requirements must be considered in crystallization plants for LiCl?
Due to the high solubility of LiCl and the associated boiling point elevations, evaporation-crystallization must always be operated at high temperatures. This results in a decrease in the viscosity of the solution, which benefits the crystallization behaviour.
Is it possible to crystallize LiCl at low temperatures, like in cooling crystallization?
Indeed, the solubility of LiCl decreases with lowering temperature. If the initial conditions of the solution allow it, it is therefore in principle possible to carry out a cooling crystallization with direct cooling. However, a larger part of the lithium chloride remains in the reject solution.
A vacuum cooling crystallization of LiCl is not economically feasible due to the high boiling point elevation.
What materials are used in crystallization plants for LiCl?
Due to the high aggressiveness of LiCl solution, resistant, high-quality materials must be chosen. This is also necessary because the produced crystals generally need to have very high purity.
Other Applications