Crystallization of Na₂SO₄ or Glauber’s Salt

Especially in industrial wastewaters, such as those from flue gas desulfurization plants, sodium sulfate is one of the components. In many cases, it is necessary to crystallize the sodium sulfate from the wastewater in order to ensure unproblematic discharge while considering regulatory requirements. But also in other processes, such as in the viscose fiber industry, sodium sulfate is separated from the overall process.
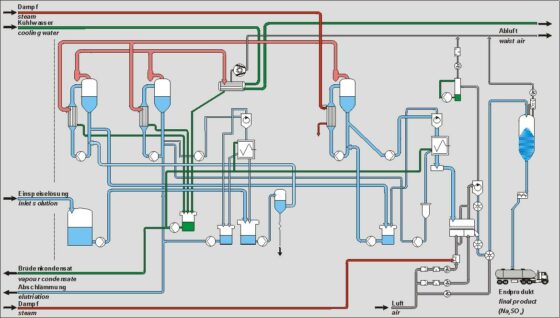
Depending on the desired end product, whether sodium sulfate or Glauber’s salt, different processes are chosen.
Evaporation Crystallization of Sodium Sulfate (Na₂SO₄)

If sodium sulfate (Na₂SO₄) is to be crystallized anhydrous, the temperature must be higher than the transformation point to the decahydrate (Glauber’s salt). The solution is evaporated to the point where the saturation limit is exceeded, and the salt precipitates.
Due to the low boiling point elevation of sodium sulfate, the process of mechanical vapor recompression has mainly established itself as the main method in recent times.

Cooling Crystallization of Glauber’s Salt (Na₂SO₄ 10H₂O)
If sodium sulfate is to be removed from the process stream as Glauber’s salt, crystallization must take place below the transformation point. This means that crystallization must occur at a temperature of about under 32°C, depending on the overall components of the solution.
The advantage of cooling crystallization with Glauber’s salt, especially in process wastewaters, is that a large amount of water is always removed from the solution and incorporated into the crystal. Thus, it is possible to reduce not only the sodium sulfate stream but also the volume of wastewater through the water incorporated into the crystal.
In practice, the crystallization of Glauber’s salt is mostly carried out in vacuum cooling crystallization plants. Here, evaporation of water is induced by lowering the pressure, which ultimately lowers the temperature of the solution.
Especially in the viscose fiber industry, it has become standard that the resulting vapor is condensed directly into sulfuric acid in mixed condensers.

Due to the high boiling point increase or vapor pressure reduction of sulfuric acid, it is possible to cool the sodium sulfate solution to very low temperatures, so that there is only a very low residual solubility of sodium sulfate in the solution.
Alternative methods include lowering the temperature of the solution by compressing the resulting vapor using steam jet apparatus or the increasingly used variant of condensation by a refrigerant produced by refrigeration machines.

EBNER – your competent partner in plant engineering
Due to many years of experience in various industrial sectors and with various solutions, EBNER is your contact for optimally executing the process design, planning, delivery, and erection according to your needs.
Frequently Asked Questions
Is it more sensible to crystallize the sodium sulfate as Na₂SO₄ or as Glauber’s salt?
This ultimately depends on the starting solution as well as the desired processing of the salt.
If the salt is to be saleable, dry Na₂SO₄ without crystal water is crystallized in an evaporation crystallization plant. This is then used, for example, in the detergent industry.
If the wastewater load is to be reduced and the salt is to be waste salt, Glauber’s salt (Na₂SO₄·10H₂O) is crystallized in a cooling crystallization plant. Thus, the volume of wastewater is further reduced by the water incorporated into the crystal.
What types of plants are used for the crystallization of sodium sulfate?
Typically, evaporation crystallization through mechanical vapor recompression, thermal vapor recompression, or as a multi-effect plant is used.
What types of plants are used for the crystallization of Glauber’s salt?
Here, vacuum cooling crystallization or crystallization by direct cooling is suitable.
Other Applications