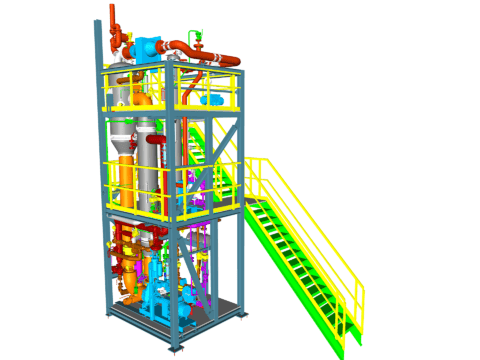
Evaporation of CaCl₂ Solution

Similar to the evaporation plant for MgCl₂ solutions, plants for concentrating CaCl₂ solutions are limited by the thermodynamic properties of the solution.
This involves considering not only a high boiling point elevation towards the saturation limit but also the viscosity of the solution when designing evaporation plants. The main reason for this is the high solubility of calcium chloride.
Therefore, in industrial practice, a steam-heated plant with a few effects is generally chosen, which is typically carried out in a counter-current process.
Due to the strong tendency to corrosion caused by the high chloride load, efforts are made to operate in as low a temperature range as possible.
A common further processing of the solution is the production of calcium chloride hydrates – usually as calcium chloride hexahydrate (CaCl₂ 6H₂O).
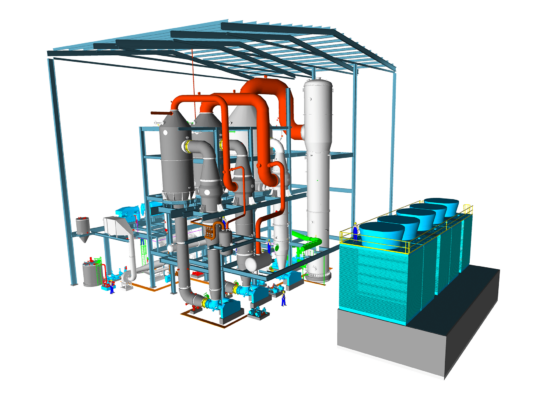
This is done by applying the hot calcium chloride solution to a cold surface, as in flaking.
Due to the lower solubility of calcium chloride at lower temperatures, local crystallization occurs. The absorption of water into the crystal then leads to local supersaturation, causing – like a chain reaction – further calcium chloride hexahydrate to form.


EBNER – your competent partner in plant engineering
With many years of experience in various industries and with different solutions, EBNER is your contact for optimally executing process design, planning, delivery, and erection according to your specific needs.
Frequently Asked Questions
Can CaCl₂ also be crystallized in a different crystal structure?
Although evaporation plants for CaCl₂ solution and crystallization can also be designed to produce, for example, CaCl₂ 2H₂O, this is not common in industrial practice.
What materials are chosen for the components of the plant?
The main components of the plant (pumps, heat exchangers, apparatus) are made from high-quality materials.
The exact choice of material depends on other accompanying substances in the solution. Possible combinations include rubber-lined steel, aluminum-bronze alloys, high-quality stainless steels or titanium.
Other applications